Chemical Composition of Matter
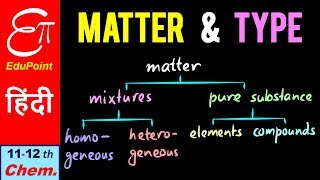
As like physical composition of matter, chemical composition is also divided into various type for better clarifications which deals with the chemical property of any substance. On this basis element is divided into three groups element, compound and mixture.
Element:
• It is a fundamental matter which can not be divided or decomposed into two or more component having different characteristics or properties by any physical or chemical process.
• Purest form of matter.
• It is of two types – metals and none metals.
• Their atoms have same nuclear charge.
• Metals are malleable in nature and good conductor of heat and electricity while non metals are brittle in nature and bad conductor of heat and electricity.
Compound:
• It is a substance formed by the chemical combination of two or more elements composed in a definite ratio.
• The physical and chemical property of the formed compound varies from the constituents or components of the earlier elements.
• For ex –(H 2 O ) water is formed from hydrogen and oxygen.
( C O 2 ) carbon dioxide is formed from carbon and oxygen.
Mixture:
• It is a substance formed by the two or more element by the means of physical combination into two or more pure element.
• They don’t have used elements of definite proportion.
• For ex – Air ( which Is mixture of many gases) , Brass ( copper + zinc) etc.